Commentary by Timo Kanninen, Founder and CSO, BC Platforms
Hypothesis
The highest priority in the COVID-19 pandemic is to ensure that enough hospital and intensive care capacity is available to treat patients as needed. This can be done by ”flattening the peak’ e.g. by radically limiting physical human interaction (lockdown) and increasing hospital capacity. This approach has worked to defend against the first wave, but as lockdown has significant consequences for the economy as well as health and social problems, different strategies for opening full lockdown but still managing the hospital capacity has been proposed for the second and following waves.
In addition to current "test, track, isolate and treat" strategies, the following high potential alternatives (game changers) have been proposed: a) vaccine or treatment breakthrough, b) breakthrough in “epinomics” and c) precise predictions of highest risk individuals (1).
Human genetics can be applied to provide precise predictions for significantly faster exit and for fact based vaccination and drug prioritisation.
Many virus-caused diseases, for example Dengue fever, have significant genetic predispositions (in humans, not in the virus), explaining why some patients require hospitalisation or intensive care, while others survive with mild or perhaps no symptoms at all. For example, this is proven for Dengue fever, according to results of the European-wide research program (EU FP7) DENFREE project (2).
Preliminary Research Findings
Evidence for strong role of genetics is piling up. One of the earliest published findings were made by TwinsUK biobank comparing COVID-19 symptoms on monozygotic and dizygotic twins (3). International COVID-19 Host Genetics Initiative recently published its first results proposing genes in chromosomes 9 and 3 contributing to COVID-19 disease severity (4). In the UK, agencies recently decided to invest £28M to recruit and produce DNA genome sequence of 20 000 patients currently or previously in intensive care due to the coronavirus and up to 15 000 patients who had mild or moderate symptoms (6). In addition to disease severity, genomics can also influence which individuals can avoid infection, for example due to mutations in ACE2 receptor (5, 7), or other direct biological reason e.g. as in Pandemrix-induced narcolepsy case (8).
In addition to genetics, many other factors contribute, but with current knowledge it seems that it would not be possible to make a good model without involving genetics. Model accuracy has direct correlation to how much isolation is needed and how fast exit can be executed. Instead of developing own population specific models from scratch, it would be possible to confirm and adjust models developed in other populations using relatively small numbers of local subjects.
Based on initial results, it is possible that host genetics will play a major role in the severity of the disease in each individual, as well as understanding which individuals are likely to be infected. It is anticipated that a genetic based prediction model to accurately identify high-risk patients requiring hospitalisation or intensive care in case of COVID-19 virus infection will be available soon. Such a model would offer a high potential alternative (game changer) to efficiently exit from lockdown. Even while the genetic model and all details are not available yet, now is the time to start thinking and planning how to adopt the prediction model in practice, when it becomes available.
Proposed Methodology to Utilize Prediction Model
Using proven, scalable, low-cost genotyping technology, the high risk citizens genetically predisposed to have severe symptoms, if affected by COVID-19, could be identified. Instead of locking down full populations, we could choose to let the disease run its course in the very-low-risk population while allocating economic and social resources toward protecting and providing for those at high risk groups - such as the elderly as well as immuno-compromised populations.
Since the elderly and those with severe chronic diseases carry greater risk in normal times, regardless of their genes, genetic testing could, in the first instance, be applied primarily to working age citizens. Figure 1 below illustrates this. Based on this risk stratification, low-risk individuals would return to work normally. Genetic data from one individual can be produced for less than 50-70 EUR (to compare, one COVID-19 virus test cost up to 200 EUR).
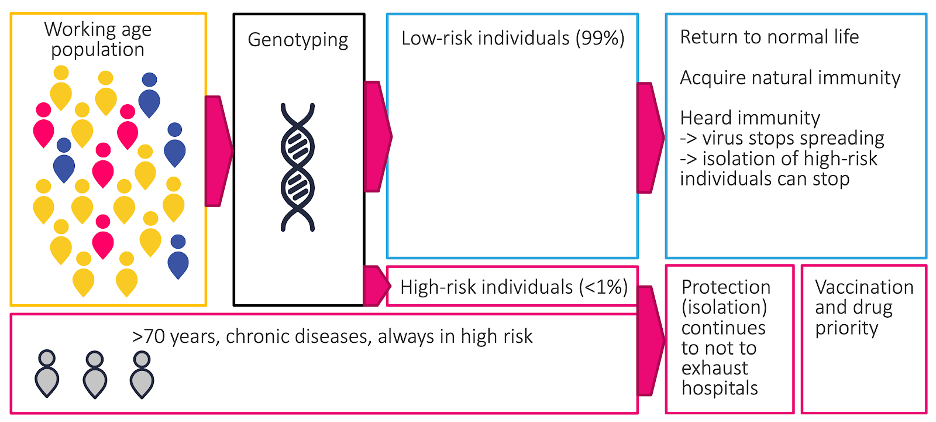
Figure 1: Approach using genetic risk model
As genetic risk is inherited, by following pedigrees it would be possible to identify a very large proportion of the high-risk subjects in the population fast, and target protection and isolation operations primarily to these subjects. When vaccinations and novel drugs become available and manufacturing ramps up, high-risk individuals would be prioritised. The proportion of high-risk subjects in the working age group is expected to be very small.
Taking DNA samples for performing genetic tests could be taken in parallel workflow with the COVID-19 test and results reported back to the individual, who, in case of high-risk status, could then alert his/her close relatives to take a genetic test, starting the chain reaction.
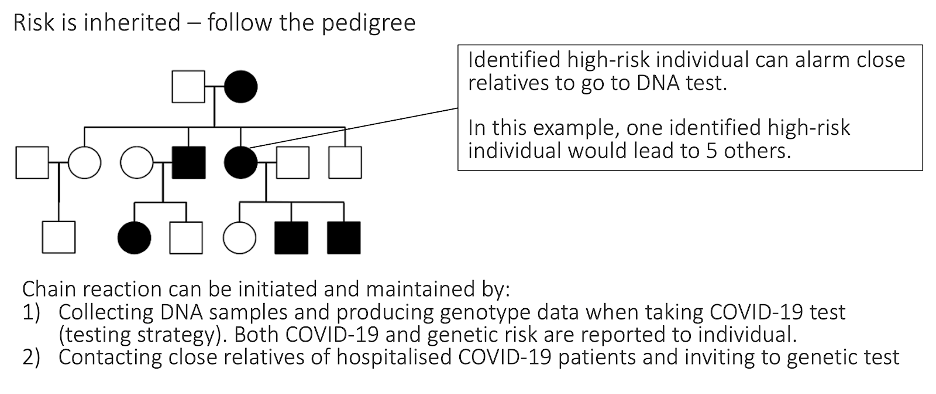
Figure 2: Identifying high-risk individuals by following the pedigrees (family history)
The next step is to identify high-risk subjects, and for this, it would be enough to produce genetic data for an estimated 15% of the working age population (this heavily depends on the population and cultural structure). Total budget of this phase would be less than 100M EUR if to use Finland as an example, compared against the estimated cost of one lockdown day (175M EUR) or continuous population wide COVID-19 testing (>1,000M EUR).
By using the above-mentioned 'follow the pedigrees' approach, a relatively small number of subjects would need to be genotyped for addressing the COVID problem. The 15% suggested is a very rough number and it depends on for example, how common possible high-risk variants are in the population and how well 'follow the pedigree' strategy works (this depends how families are formed, communicating, cultural factors etc).
However if there is a secondary objective such as advancing a national personalised medicine initiative, and improving healthcare quality and significantly saving healthcare costs in the longer term, a larger proportion of population would be needed to be genotyped.
Longer Term Benefits
In addition to helping in acute COVID-19 lockdown exit, produced genetic data could be used for predictive, preventive and personalised healthcare for preventing and delaying chronic diseases, improving healthcare quality and significantly lowering healthcare costs. Later, genetic data production could be expanded to cover the whole population for maximum benefits.
This pandemic also underlines the importance of having population level biobanks and national PMI programs with genetic data already available for fighting possible future epidemics and Life Science research work in general. All nations must understand their own genome to utilise the promise of genomics in healthcare.
References:
- Boston Consulting Group: COVID-19: Win the Fight, Win the Future https://www.bcg.com/publications/2020/covid-scenario-planning-winning-the-future-series.aspx
- DENFREE final report: https://cordis.europa.eu/project/id/282378/reporting
- The Guardian: Study of twins reveals genetic effect on Covid-19 symptoms https://www.theguardian.com/world/2020/apr/27/study-of-twins-reveals-genetic-effect-on-covid-19-symptoms
- The New York Times:Genes May Leave Some People More Vulnerable to Severe Covid-19 (https://www.nytimes.com/2020/06/03/health/coronavirus-blood-type-genetics.html?smid=nytcore-ios-share), https://www.covid19hg.org
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7079879/
- Illumina, UK Agencies Partner on £28M Sequencing Study of COVID-19 Patients https://www.genomeweb.com/sequencing/illumina-uk-agencies-partner-28m-sequencing-study-covid-19-patients?utm_source=Sailthru&utm_medium=email&utm_campaign=GWDN%20Wed%20AM%202020-05-13&utm_term=GW%20Daily%20News%20Bulletin#.XrwpUi97F60
- https://www.ersnet.org/covid-19-blog/ace2-receptor-blockers--a-novel-therapeutic-approach-for-covid-19
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6413474/
Prepared by : Timo Kanninen and Team BC Platforms, 20 May 2020, updated 4th of June 2020




